Chemistry model salt molecule diatomic sodium chlorine NaCl scientific element formula
Chlorine forms a series of oxides (Table 10.3. 2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides are summarized in Table 10.3. 2. While, the oxides of chlorine are not very stable (in fact several are shock sensitive and are prone to explode) the conjugate oxyacids are stable.

Chlorine Cl (Element 17) of Periodic Table NewtonDesk
The atomic number of this chemical element is 17. It appears as a pale yellow-green gas. Liquid chlorine can cause skin burn and chlorine in its gaseous form irritates the mucous membrane. Its position as per the periodic table is between fluorine and bromine. Its electronic configuration is [Ne]3s23p5.
"Chlorine Molecule" by erzebetth Redbubble
Molecular Formula Cl Average mass 70.906 Da Monoisotopic mass 69.937706 Da ChemSpider ID 22933 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 7782-50-5 [RN] Bertholite Chloor [Dutch] Chlor [German] [ACD/IUPAC Name]
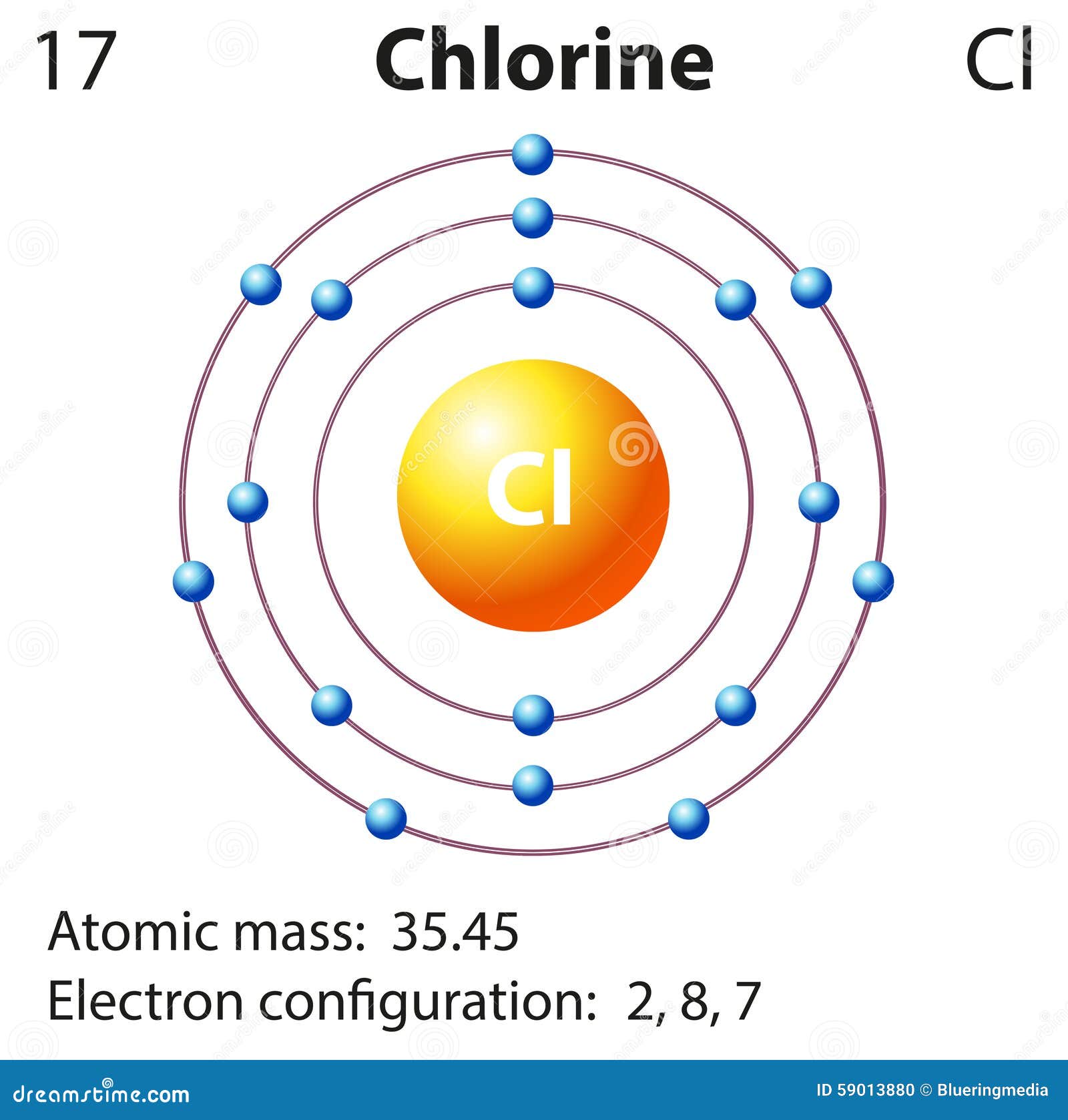
Diagram Representation Of The Element Chlorine Stock Illustration Image 59013880
1. Determine the total number of valence electrons in the chlorine molecule. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell. Therefore, the total number of valence electrons= 7 (2)= 14. 2.

Chlorine Benefits, Chemical Safety of Chlorine, Properties & Facts
Please enable Javascript in order to use PubChem website. Chlorine | Cl2 | CID 24526 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.

"Chlorine Molecule" Tshirt by EvasDreams Redbubble
Let us follow some steps to draw the Lewis structure of chlorine dioxide: Step 1: Find the total valence electrons in one molecule of chlorine dioxide. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. There are two oxygen molecules in chlorine dioxide so the total is 19.

Aufbau Diagram For Chlorine
Element Chlorine (Cl), Group 17, Atomic Number 17, p-block, Mass 35.45. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
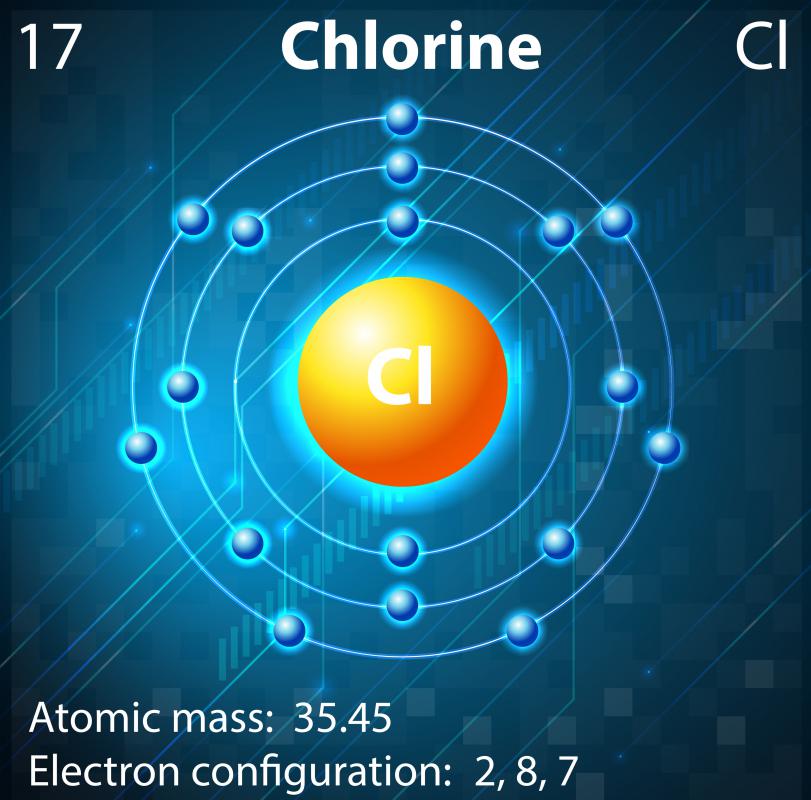
What Are the Properties of Chlorine? (with pictures)
Formula: Cl 2 Molecular weight: 70.906 IUPAC Standard InChI: InChI=1S/Cl2/c1-2 IUPAC Standard InChIKey: KZBUYRJDOAKODT-UHFFFAOYSA-N CAS Registry Number: 7782-50-5 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .

Chlorine Pentafluoride Lewis / Chlorine pentafluoride is a square pyramidal molecule. Ajor Png
Chlorine atoms are covalently bonded to form a diatomic Cl 2 molecule. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. Table of Contents How to draw Lewis Structure for Cl 2 Molecular Geometry of Cl 2 Hybridization of Cl 2 Polarity of Cl 2 Frequently Asked Questions - FAQs

An Atom of Chlorine Diagram Stock Vector Illustration of structure, chemistry 141346692
For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair.

Draw the electron dot structure of chlorine molecule Brainly.in
Atomic Structure. Why does a Chlorine Molecule have a Covalent Bond?. Chlorine is a non-metal. A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the periodic table. Two chlorine atoms will each share one electron to get a full outer shell and form a stable Cl 2 molecule.. This is a picture of the shared electrons making a covalent bond in a chlorine molecule.
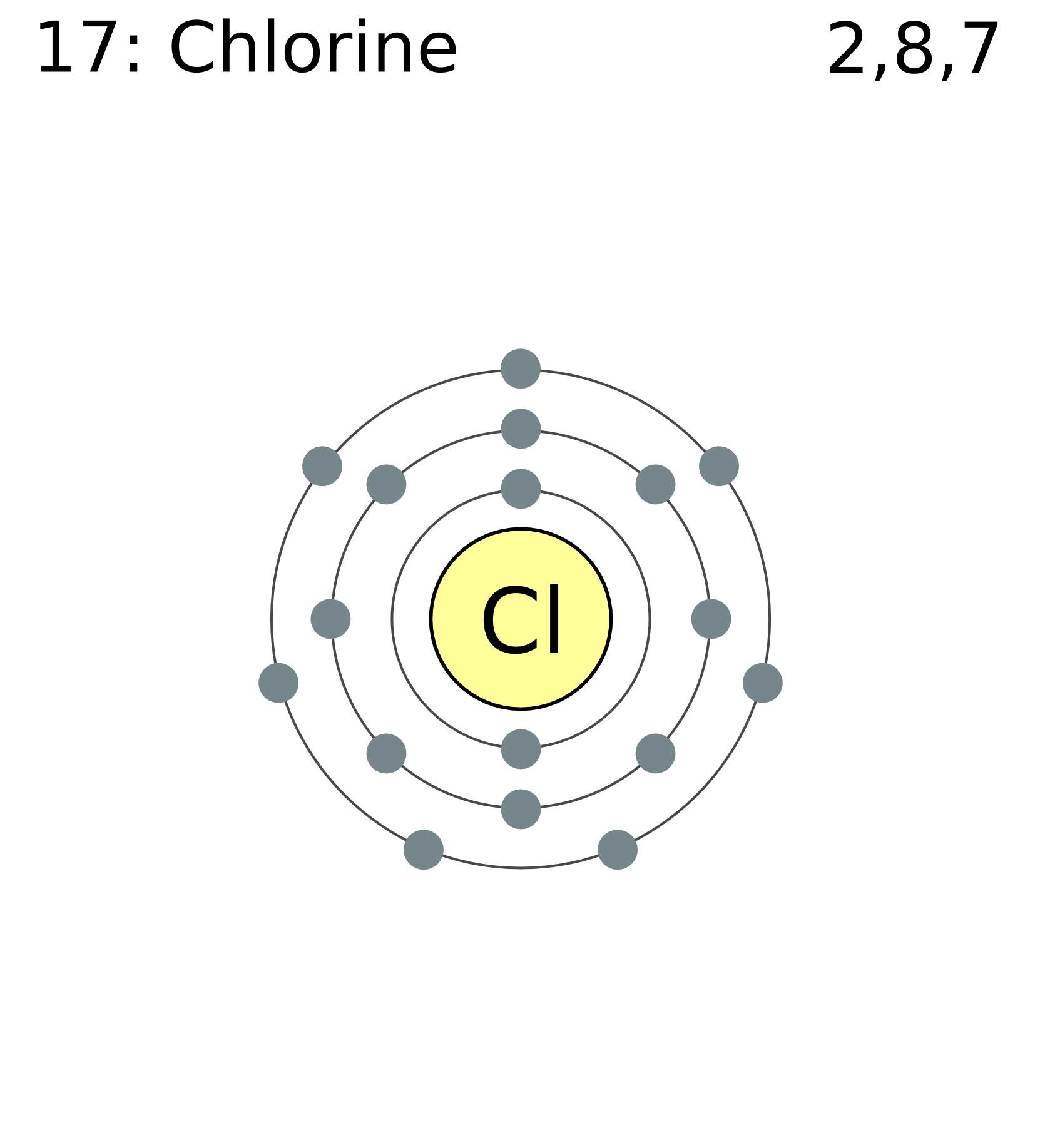
FileElectron shell 017 chlorine.png Wikimedia Commons
The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration.. Step 2: Draw a skeleton structure of the molecule, arranging the atoms around a central.

Atom chlorine Royalty Free Vector Image VectorStock
Exercise 3.3.4.3 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.

Draw the electron dot structure of chlorine molecule Brainly.in
The structure shown above, which is a chemically-correct representation of a covalent compound, is a Lewis structure that represents the molecule that is formed when fluorine and sulfur bond with one another. Example \(\PageIndex{1}\) Draw the Lewis structure that represents the compound that is formed when carbon and chlorine bond with one.

Chlorine wikidoc
To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number.
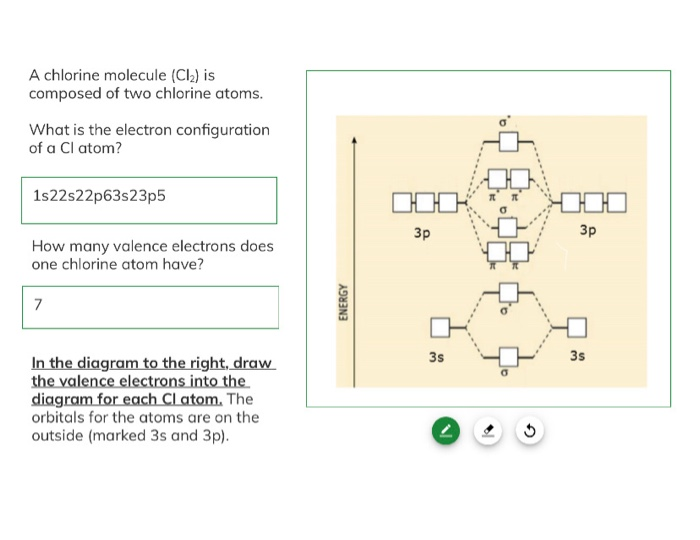
Solved A chlorine molecule (Cl) is composed of two chlorine
A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure (Chlorine gas).For the Cl2 structure use the periodic table to find the total number of.